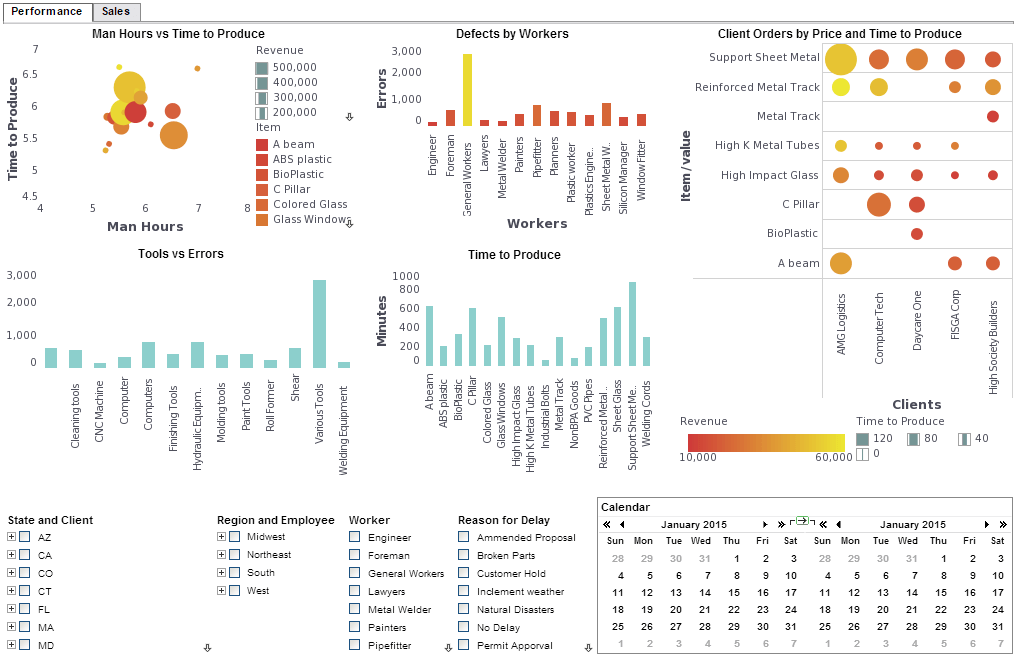
Evaluate InetSoft's Customizable Report Tool
The customizable report is an important concept in Style Intelligence. We have shown three ways to pass parameters to a report, either interactively or through static configuration files. In subsequent sections, we will demonstrate more ways to pass parameters and handle the requests. Since the replet architecture automates the entire parameter prompting and submission process, all a replet needs to do is declare the parameters it needs and build a report object accordingly. This provides the report developers with a powerful tool to create sophisticated custom reports.
As a generic API, the parameterization support can be used in any way a programmer chooses. However, there is a danger of overusing this feature and making the report too complicated to access. The following are some guidelines to help create more consistent and easy to use interactive reports.
• Initialization parameters should be used to configure static information. Normally, they should not be used to change the basic content of the report. Some obvious examples are parameters for a database connection, cloud server locations and report template resource names.
• Creation parameters should consist of a relatively small number of options. If there are many configurable options on the report, most of them should be presented as Customized parameters. By keeping the creation parameters simple, users can quickly open a report without going through a large number of choices. If they choose to customize the report later, they can activate the customizer explicitly. This approach is less intrusive and more user friendly.
• Customization parameters should be used for dynamically changing report element attributes. This method provides a consistent and predictable way to customize a report.
Replet Interactions
The replet interactions are based on the distributed event-handling model. The BasicReplet API provides a few high-level methods for adding common interactions, such as hyper-links and popup menus. A programmer can also use the generic event handling API to add custom event handling to a replet.
All user interactions follow the same sequence:
1. A user action on the viewer generates an event.
2. The event is passed to the event handling routine. Simple events are handled in a client. If the client does not handle the event, the event is forwarded to the replet event handling routine over the network.
3. When an event is processed, one or more commands are returned from the event handling routine.
4. The command is executed on the client. If a command generates another event, the same process is repeated. Otherwise, the viewer returns to the waiting state.
Case Study: VigilantPharma - Leveraging a Customizable Report Tool in Pharmacovigilance
VigilantPharma is a global pharmacovigilance (PV) firm dedicated to monitoring the safety of pharmaceutical products and ensuring that any potential risks are identified, assessed, and mitigated. Established in 2008, VigilantPharma partners with pharmaceutical companies, biotechnology firms, and regulatory authorities to collect, analyze, and report adverse drug reactions (ADRs) and other drug safety data.
Pharmacovigilance is a critical function in the pharmaceutical industry, focusing on the detection, assessment, and prevention of adverse effects or any other drug-related problems. With the increasing complexity of global drug safety regulations and the need for real-time monitoring of drug safety data, VigilantPharma faced several challenges in managing and reporting data effectively.
To address these challenges, VigilantPharma implemented a Customizable Report Tool that enabled the firm to streamline its reporting processes, enhance data accuracy, and improve decision-making across its operations.
Challenges Faced by VigilantPharma
- Data Complexity and Volume:
- The firm needed to manage and analyze vast amounts of data from multiple sources, including clinical trials, post-marketing surveillance, and spontaneous reports from healthcare professionals and patients. This data included various formats, making it challenging to integrate and analyze effectively.
- Regulatory Compliance:
- Pharmacovigilance firms operate under strict regulatory requirements, such as those set by the FDA, EMA, and other global health authorities. Ensuring timely and accurate reporting of ADRs while maintaining compliance with these regulations was a significant challenge for VigilantPharma.
- Timely Reporting:
- VigilantPharma needed to provide timely and accurate reports to pharmaceutical clients and regulatory bodies. Delays or inaccuracies in reporting could lead to regulatory penalties, product recalls, or harm to patients.
- Custom Reporting Needs:
- Different stakeholders, including clients and regulators, had varying requirements for report formats, data fields, and analysis metrics. VigilantPharma needed a flexible tool that could accommodate these diverse reporting needs without requiring extensive manual effort.
- Collaboration Across Teams:
- The firm's global operations required seamless collaboration between teams in different locations, including data entry personnel, safety scientists, and regulatory affairs experts. Sharing and reviewing reports efficiently was critical to ensuring a coordinated response to safety signals.
Solution: Implementation of a Customizable Report Tool
To overcome these challenges, VigilantPharma implemented a Customizable Report Tool that provided the flexibility, automation, and integration capabilities necessary for effective pharmacovigilance reporting. The tool allowed VigilantPharma to generate, manage, and share reports tailored to the specific needs of different stakeholders, all while ensuring compliance with regulatory standards.
Key Features of the Customizable Report Tool
- Data Integration and Standardization:
- The tool integrated data from multiple sources, including EHRs, clinical trial databases, and post-marketing surveillance systems. It standardized this data, allowing for seamless analysis and reporting.
- The tool also supported the import and export of data in various formats, ensuring compatibility with different client and regulatory systems.
- Custom Report Templates:
- The tool provided a library of customizable report templates that could be tailored to specific reporting requirements. These templates allowed users to select relevant data fields, apply filters, and format reports according to regulatory or client needs.
- Users could also create and save their own templates, reducing the time required to generate routine reports.
- Automation of Reporting Workflows:
- The tool automated key reporting workflows, such as the generation and submission of periodic safety update reports (PSURs) and individual case safety reports (ICSRs). Automated scheduling ensured that reports were generated and delivered on time, reducing the risk of regulatory non-compliance.
- Alerts and notifications were built into the system to ensure that stakeholders were informed of upcoming deadlines or potential issues.
- Real-Time Analytics and Dashboards:
- The tool included real-time analytics and interactive dashboards that allowed VigilantPharma to monitor safety signals, track ADR trends, and assess the overall safety profile of drugs. These dashboards provided both high-level summaries and detailed drill-down views, enabling quick identification of emerging risks.
- Data visualization tools, such as charts and graphs, helped stakeholders understand complex data more easily.
- Role-Based Access and Security:
- The tool provided role-based access controls, ensuring that only authorized personnel could view or edit specific reports. This was particularly important for maintaining data privacy and security in line with regulatory requirements.
- Audit trails were also included, allowing VigilantPharma to track who accessed or modified reports, further enhancing accountability and compliance.
- Collaboration and Sharing:
- The tool facilitated collaboration by allowing users to share reports and data securely with team members, clients, and regulatory bodies. Built-in commenting and review features enabled efficient communication and ensured that all stakeholders were aligned before reports were finalized.
- Reports could be shared in various formats, including PDF, Excel, and XML, depending on the needs of the recipient.
Benefits of Using the Customizable Report Tool
- Improved Regulatory Compliance:
- The tool helped VigilantPharma maintain compliance with global pharmacovigilance regulations by automating reporting workflows and ensuring that reports were accurate and delivered on time. This reduced the risk of penalties and enhanced the firm's reputation with regulatory authorities.
- Enhanced Data Accuracy and Consistency:
- By integrating and standardizing data from multiple sources, the tool improved the accuracy and consistency of reports. This ensured that VigilantPharma's clients received reliable information to support their decision-making.
- Increased Efficiency:
- Automation of routine reporting tasks freed up valuable time for VigilantPharma's safety scientists and regulatory experts. This allowed them to focus on more complex analyses and proactive risk management, rather than being bogged down by manual data entry and report generation.
- Tailored Reporting for Diverse Stakeholders:
- The customizable report templates enabled VigilantPharma to meet the specific needs of different clients and regulators without requiring extensive manual effort. This flexibility improved client satisfaction and allowed the firm to serve a broader range of customers.
- Proactive Safety Monitoring:
- The real-time analytics and dashboards provided VigilantPharma with the tools to proactively monitor drug safety and detect emerging risks early. This enabled the firm to take swift action when needed, potentially preventing harm to patients and avoiding costly product recalls.
- Enhanced Collaboration and Communication:
- The collaboration features of the tool improved communication between teams and stakeholders, ensuring that everyone was aligned and informed. This led to more efficient workflows and better coordination across the firm's global operations.
Challenges Encountered
While the implementation of the Customizable Report Tool brought significant benefits, VigilantPharma faced some challenges during the process:
- Initial Data Integration:
- Integrating data from multiple, disparate sources was complex and required significant effort upfront. VigilantPharma had to invest in data cleaning and standardization to ensure that the data fed into the tool was accurate and compatible.
- User Training and Adoption:
- Ensuring that all team members were comfortable using the tool required comprehensive training. Some staff initially resisted the change from manual processes, but VigilantPharma addressed this by providing ongoing support and demonstrating the tool's benefits in practice.
- Customization Complexity:
- While the ability to customize reports was a significant advantage, it also introduced complexity. VigilantPharma had to carefully manage customization to ensure that reports remained consistent and aligned with regulatory requirements.
Results and Outcomes
The implementation of the Customizable Report Tool at VigilantPharma led to substantial improvements in the firm's pharmacovigilance operations:
- Regulatory compliance was maintained at a high level, with no major reporting delays or inaccuracies reported since the tool's implementation.
- Operational efficiency increased by 25%, as measured by the reduction in time spent on manual report generation and data entry tasks.
- Client satisfaction improved, with feedback indicating that the tailored reporting capabilities and timely delivery of reports were highly valued.
- Early detection of safety signals improved, leading to more proactive risk management and fewer serious adverse events reported.